Conducting Polymers
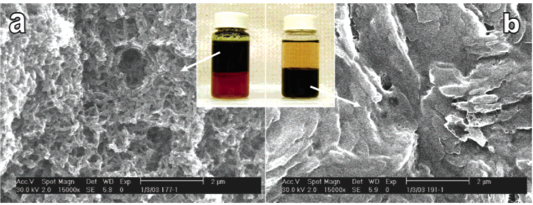
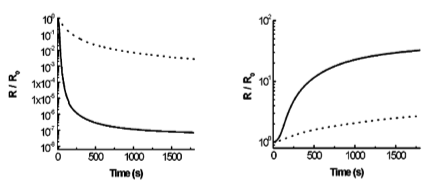
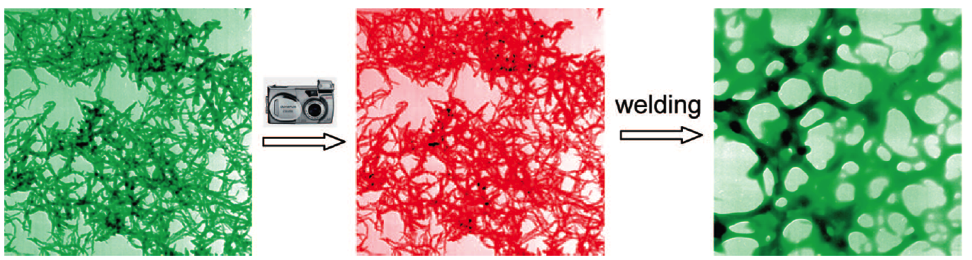
Our lab has conducted the pioneered research on the synthesis of polyaniline nanofibers since year of 2003. We found out that polyaniline nanofibers can be synthesized through facile interfacial polymerization or rapid mixing without stirring. In contrast to the traditionally formed polyaniline, the as-synthesized polyaniline nanofibers have a high surface area, resulting in much higher sensitivity in chemiresistors upon exposures to acidic or basic environments. Polyaniline nanofibers, due to its low thermal conductivity and high efficiency in the photothermal conversion, form covalent bonding between polymer chains upon exposure to a flash light. This photo welding process generates smooth but holey surfaces. Furthermore, the heat generated is also able to melt and/or form bonding with additives, forming versatile composites.
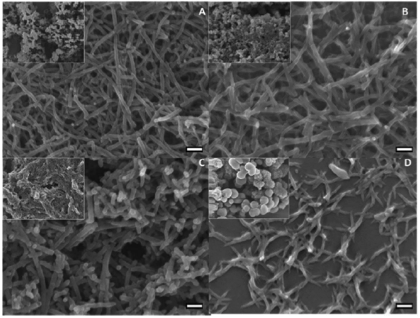
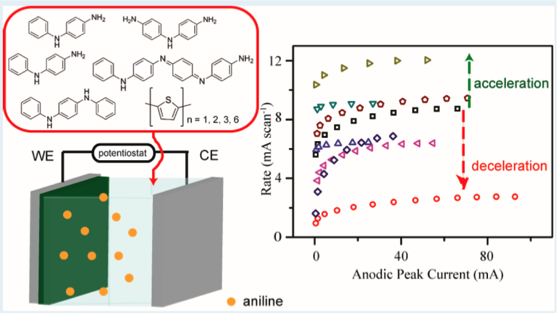
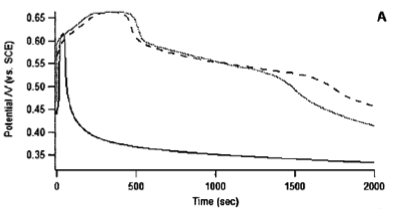
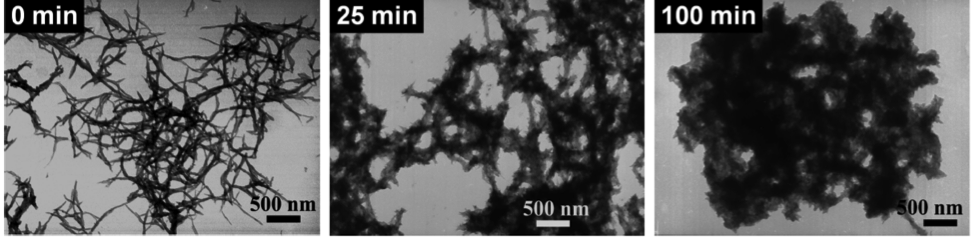
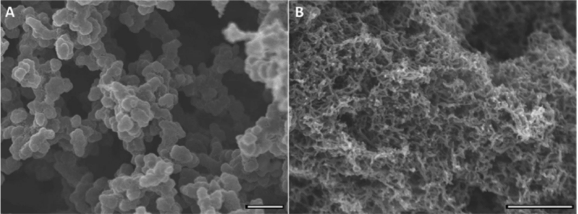
In light of polyaniline nanofibers, our group has investigated the morphologies of polymers formed by various aniline derivatives and under different chemical environments. It was concluded that oligomers are considered seeds or catalysts to increase the speed of polymerization processes, even in the systems of pyrrole and thiophene as well. The accelerated process can be monitored by the open circuit voltage, reaction temperature, and the amount of polyaniline deposited on electrodes. The acceleration of polymerization also results in more fibrillary and nanosized morphologies instead of agglomerated chunks.
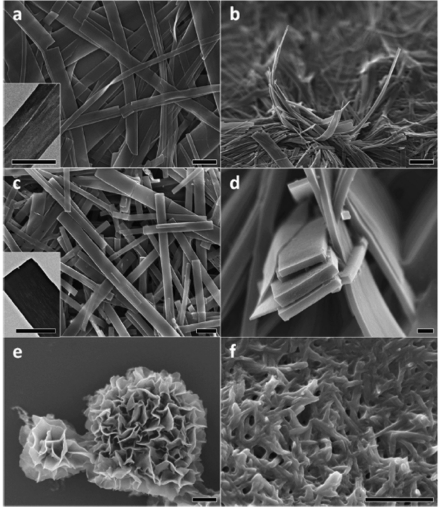
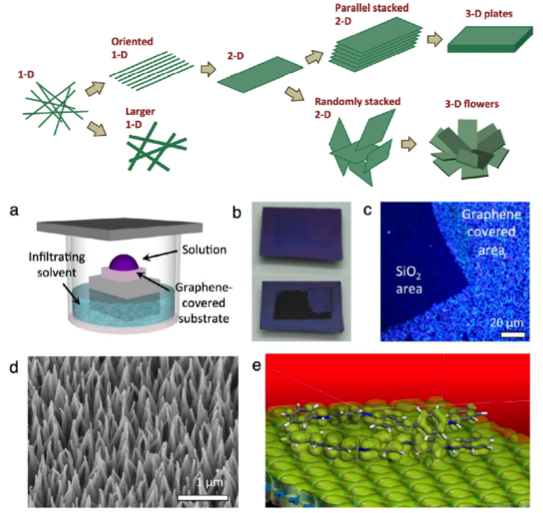
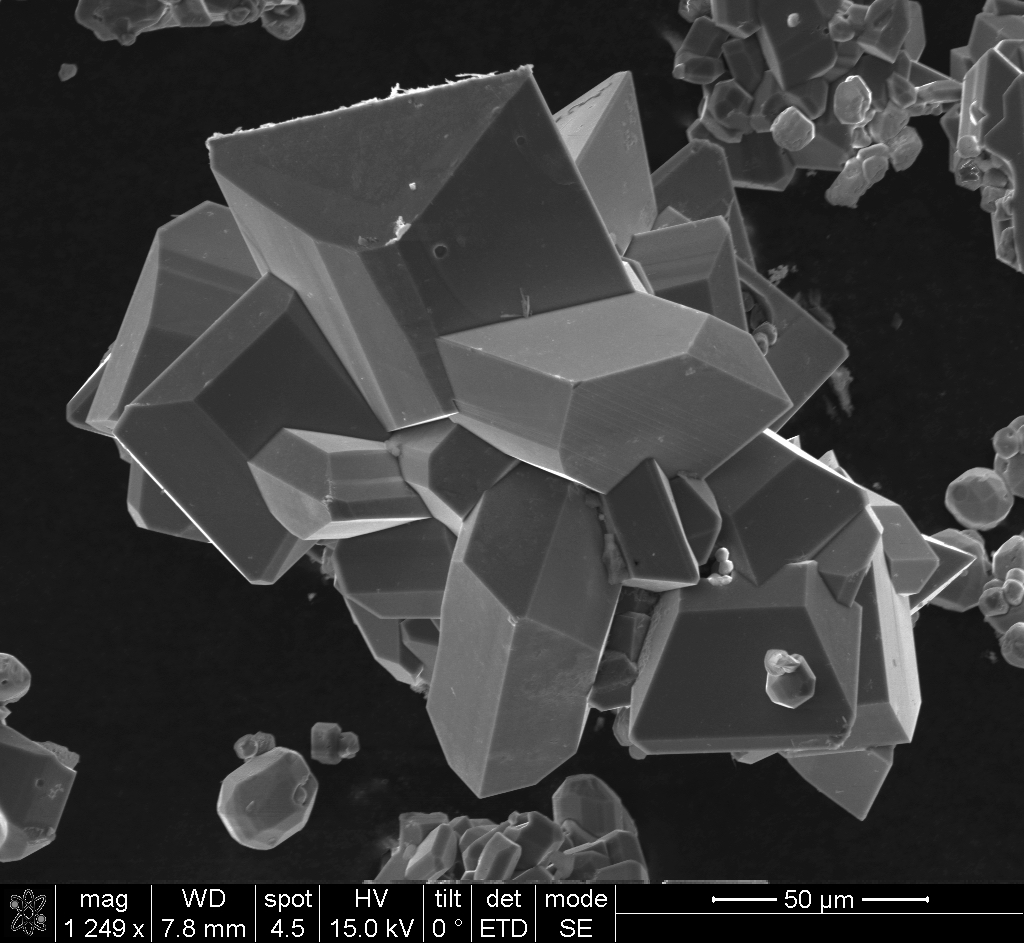
Aniline tetramer, as the smallest repeating unit of polyaniline, has attracted our attention. The bottom-up hierarchical assembly evolution was observed by doping aniline tetramers with different types of acids with various concentrations. Those highly crystalline crystals can be observed under transmission electron microscopes and show nice diffractions for elucidating the crystal structures. By using graphene as the substrate, aniline tetramer is found to form vertically aligned crystals via a non-solvent infiltration crystallization method due to the pi-pi stacking between graphene and tetraaniline. The “standing” crystal makes it possible to measure the interchain electrical conductivities. Lately, we have focused on the investigation of physical and chemical properties of tetraaniline, and the possible applications such as water filtration membranes and energy storage devices.